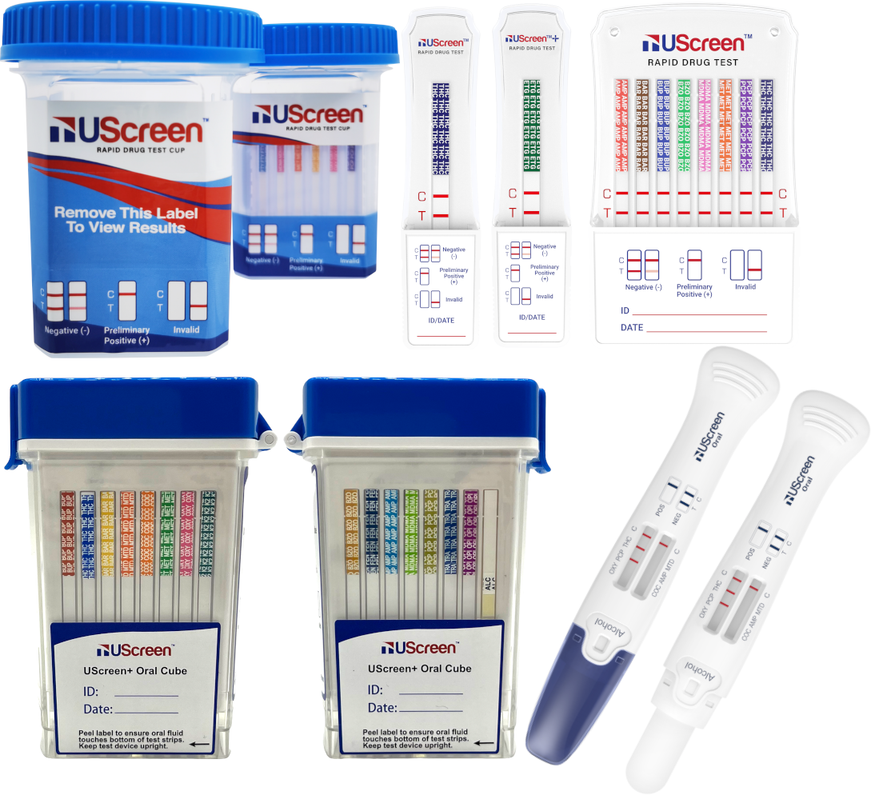
UScreen® Rapid Drug Test Cups — Reliable, Fast, and CLIA-Waived Screening Solutions
Why Accurate Drug Testing Matters
UScreen® Rapid Drug Test Cups — Proven Performance, Trusted Results
✅ CLIA-Waived ✅ FDA Cleared ✅ Tamper-Resistant ✅ Same-Day Shipping
Shop UScreen® Now | or call (800) 915-7017
In occupational health, substance abuse prevention, and clinical diagnostics, choosing the right rapid testing device can save time, protect compliance, and reduce costs.
At Speares Medical, we specialize in professional-grade testing supplies that combine accuracy, speed, and ease of use — none more trusted than the UScreen® Rapid Drug Test Cup.
The UScreen® line, developed by TransMed Co, LLC, delivers consistent, laboratory-quality screening results in a simple, self-contained cup. Available in multiple panel configurations, including 5, 10, 12, 13, 14, 15, 16, 17, 18, 19, 20 and 22-panel versions, it meets the needs of both small clinics and large workplace programs.
What Is the UScreen® Rapid Drug Test Cup?
The UScreen® Drug Test Cup is a one-step lateral flow immunoassay designed for the qualitative detection of drugs and metabolites in human urine. It provides rapid results (within 90 seconds) and eliminates the need for pipetting, mixing, or exposure to the specimen.
Core Features
- Multi-panel detection of up to 22 substances — including THC, COC, OPI, AMP, BUP, MTD, FYL (Fentanyl), and K2.
- Built-in adulteration / specimen-validity tests (pH, SG, CR, OX) to detect tampering.
- Results in under 5 minutes — negative results may appear in as little as 90 seconds.
- FDA 510(k) Cleared and CLIA Waived for employment and insurance testing.
- Zero-exposure design — donor provides specimen directly into a sealed cup.
For complete product options, visit the UScreen® Collection at Speares Medical.
Benefits of Using the UScreen® Cup
1. Rapid Results and Efficiency
Compared to laboratory testing that may take 24–48 hours, the UScreen® Cup provides clear results on-site within minutes — perfect for pre-employment or random workplace testing.
2. Comprehensive Substance Detection
UScreen® offers configurations from 4 to 22 panels to meet changing testing requirements.
The 16-Panel and 22-Panel models detect traditional drugs and emerging synthetics such as fentanyl, K2/spice, and Tramadol.
→ View our UScreen® 22-Panel Premium Cup for advanced coverage.
3. Specimen Validity and Adulteration Protection
Optional built-in adulterant pads measure creatinine, specific gravity, pH, and oxidants — helping detect substituted or diluted samples.
4. Simple Interpretation
Results are visual:
- Two lines = Negative
- One line = Presumptive Positive
- No control line = Invalid Test
5. Compliance and Certification
Several UScreen® models are CLIA-Waived and FDA 510(k) Cleared, allowing clinics, treatment centers, and employers to perform legally defensible testing.
Typical Applications
- Pre-Employment Screening — verify candidate compliance quickly.
- Random / Post-Accident Testing — reduce liability by ensuring workplace safety.
- Clinical Monitoring — monitor patients in pain-management or rehabilitation programs.
- Probation and Parole — on-site testing with confirmatory lab follow-up when required.
- Mobile or Remote Screening Events — lightweight, self-contained design ideal for travel.
Implementation Best Practices
- Select the correct panel configuration for your testing program.
- Store between 36°F and 86°F (2–30°C); avoid freezing.
- Observe temperature strip within 4 minutes of collection.
- Read results at 5 minutes; do not interpret after 10 minutes.
- Confirm positives via GC/MS or LC/MS in accordance with your policy.
- Document chain of custody when used for employment or legal testing.
Downloadable quick-reference instructions are available on the Speares Medical Resources Page for staff training and compliance.
FAQs
How accurate is the UScreen® Cup?
UScreen® rapid tests are > 99% accurate at the established cut-off levels when used correctly. For regulatory programs, presumptive positives should be confirmed via a certified lab.
Which substances can be tested?
Depending on configuration: AMP, BAR, BUP, BZO, COC, ETG, FYL, K2, MDMA, MET, MOP, MTD, OXY, PCP, THC, TRM, and more.
→ Compare models on the UScreen® Comparison Chart.
Are results legally defensible?
Yes — when administered under a written policy with confirmatory testing and MRO review. Speares Medical provides guidance documents upon request.
How long are the results valid?
Once read properly within the specified window, results remain valid for record purposes; however, specimens should be discarded after interpretation unless sent for confirmation.
Choosing Between UScreen® Cup, Dip Card, and Oral Cube
| Product Type | Sample Type | Advantages |
|---|---|---|
| UScreen® Cup | Urine | Most comprehensive, CLIA-Waived versions available |
| UScreen® Dip Card | Urine | Compact and budget-friendly |
| UScreen® Oral Plus Cube | Saliva | Non-invasive collection, ideal for on-site or remote testing |
If you prefer a saliva-based device, explore our UScreen® Oral Plus Flip-Top Cube.
Integration With Your Testing Program
Speares Medical offers full support to integrate UScreen® products into existing compliance workflows:
- Custom training materials and chain-of-custody forms
- Sample policy templates for DOT and non-DOT testing
- Certificate of conformance and lot-number tracking
- Priority fulfillment from our U.S. warehouse
Contact our technical support team at sales@spearesmedical.com for implementation assistance or bulk pricing.
Recommended Reading
- How to Choose the Right Drug Test for Your Workplace
- What Does CLIA-Waived Mean in Rapid Testing?
- UScreen® Oral Plus vs Urine Cup — Which Is Right for You?
- FDA 510(k) Database Listing for UScreen® Cup
- SAMHSA Certified Lab Information
- TransMed Co – UScreen® Manufacturer
- American Drug Test – Distributor Partner
Recent Posts
-
UScreen® Rapid Drug Test Cups — Reliable, Fast, and CLIA-Waived Screening Solutions
Why Accurate Drug Testing Matters UScreen® Rapid Drug Test Cups — Proven Performance, Trusted Result …7th Nov 2025